Now Reading: Komprehensif Yapısal Çalışma ile Kromatin Yeniden Şekillendirme Üzerine İçgörüler
-
01
Komprehensif Yapısal Çalışma ile Kromatin Yeniden Şekillendirme Üzerine İçgörüler

Komprehensif Yapısal Çalışma ile Kromatin Yeniden Şekillendirme Üzerine İçgörüler
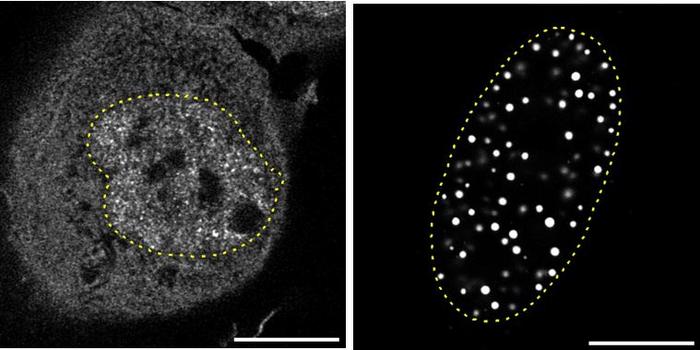
Chromatin remodeling plays a vital role in gene regulation, as it determines how DNA is packed and accessed within the cell. Recent research from St. Jude Children’s Research Hospital has shed light on this essential process, specifically examining the chromatin remodeler SNF2H. Under the leadership of Dr. Mario Halic, the research team conducted a comprehensive structural study, revealing 13 distinct structures of SNF2H. These findings illuminate the mechanisms behind nucleosome sliding—a crucial aspect of gene accessibility—offering new insights into how chromatin dynamics modulate gene expression.
The significance of chromatin remodeling cannot be overstated, particularly in the context of cellular function. Chromatin consists of DNA wound around histone proteins, forming nucleosomes that package genetic material within the nucleus. Nucleosomes are not static; they can slide along the DNA strand, which plays a critical role in regulating gene expression. Understanding the mechanical underpinnings of nucleosome sliding has remained a challenge in the field, but Halic’s team has taken a groundbreaking step to provide clarity on this complex process.
To explore the dynamics of SNF2H, the researchers employed cryo-electron microscopy (cryo-EM), a cutting-edge imaging technique that captures biomolecules in their near-native states at unprecedented resolutions. This innovative approach allowed the team to observe SNF2H interacting with nucleosomes in real-time, specifically in the presence of ATP—the energy source needed for this remodeling process. Unlike previous studies that provided static images of protein conformations, this study reveals the continuous motions of SNF2H, illustrating its dynamic role in manipulating DNA structure.
The detailed analysis of the collected data led to the identification of 13 distinct states of the SNF2H-nucleosome complex, which correspond to various moments in the nucleosome sliding process. By categorizing these states into five functional groups, the researchers pieced together the intricate choreography of the remodeling process. These comprehensive findings enhance our understanding of the relationship between chromatin structure and gene accessibility and expression.
Among the unique aspects of this research was the introduction of specific mutations and crosslinking agents designed to stabilize certain protein conformations. This strategic experimentation allowed the researchers to validate the significance of specific movements within SNF2H during its function. By resolving conflicting observations from previous studies, this research offers a more cohesive understanding of how nucleosome sliding operates, unraveling layers of complexity previously thought to be insurmountable.
Dr. Halic emphasized the importance of this research, stating, “Nucleosomes carry all the genetic information inside the nucleus of the eukaryotic cell. Chromatin remodelers help the cell access and propagate that information.” This assertion highlights not only the significance of understanding the mechanics of chromatin remodeling but also the broader implications for health and disease. Insights gained from this research could lead to new therapies targeting chromatin dynamics, potentially reversing the effects of disrupted gene expression associated with various diseases, including cancer.
The implications of SNF2H’s function extend beyond gene regulation; disruptions in the enzyme’s activity have been linked to developmental disorders. Therefore, a thorough understanding of SNF2H is essential for both basic biological research and medical applications. The findings from this study may inform therapeutic strategies aimed at restoring normal chromatin functions, representing a significant step forward in treating genetic conditions rooted in chromatin dysregulation.
Financial backing for this groundbreaking research came from notable sources, including the National Institutes of Health and the American Lebanese Syrian Associated Charities (ALSAC). Such support underscores the significance of the investigation and the potential impact of the findings on both basic research and clinical practices. Collaborative efforts in this line of research illustrate the dynamic interplay between structural biology and medicine, paving the way for an enriched understanding of cellular mechanisms that govern life.
In sum, this pioneering research marks a significant advancement in our understanding of the dynamics involved in chromatin remodeling through the actions of SNF2H. The study not only clarifies the molecular interactions at play but also sets the stage for future explorations of chromatin dynamics and their broader implications for gene regulation and cellular behavior. The advancements in imaging techniques, coupled with meticulous experimental design, speak to the promise of ongoing investigations into the intricacies of gene expression.
As research continues to probe the molecular interactions within the nucleus, scientists hold the potential to illuminate pathways connecting genetic information and cellular functionality. The insights gleaned from such studies will undoubtedly inspire subsequent investigations targeting the complexities of chromatin remodeling and its implications for therapeutic innovations aimed at managing genetic and epigenetic diseases.
As we continue to explore the structural nuances of chromatin remodelers like SNF2H, we are reminded of the elegant complexity of life at the molecular level. This research not only contributes significantly to our understanding of chromatin dynamics but also acts as a catalyst for subsequent research endeavors aimed at unlocking the potential of gene regulation.
By advancing our understanding of chromatin remodeling mechanisms, scientists are well-positioned to harness this knowledge for targeted medical interventions, enhancing our ability to address diseases stemming from uncontrolled gene expression. The trajectory established by this research paves the way for future inquiries into the role of chromatin dynamics in health and disease, ultimately contributing to the ever-evolving narrative of molecular biology.
**Araştırma Konusu**: Chromatin remodeling ve gen düzenlenmesi
**Makale Başlığı**: Comprehensive Structural Study of the Chromatin Remodeler SNF2H
**Haberin Yayın Tarihi**: 3 Nisan 2025
**Web References**: Cell Research Yayını
**Doi Referans**:
**Resim Credits**: St. Jude Children’s Research Hospital
**Anahtar Kelimeler**: Chromatin remodeling, SNF2H, nucleosome sliding, gene regulation, structural biology, cryo-electron microscopy, ATP hydrolysis, protein interactions, disease implications, developmental disorders.