Now Reading: Karaciğer Kanserinde Aurora Kinaz İnhibisyonu: Tümör Büyümesini Durdurma ve Hücre Farklılaşmasını Artırma Stratejisi
-
01
Karaciğer Kanserinde Aurora Kinaz İnhibisyonu: Tümör Büyümesini Durdurma ve Hücre Farklılaşmasını Artırma Stratejisi
Karaciğer Kanserinde Aurora Kinaz İnhibisyonu: Tümör Büyümesini Durdurma ve Hücre Farklılaşmasını Artırma Stratejisi
Liver cancer remains one of the deadliest malignancies worldwide, with hepatocellular carcinoma (HCC) constituting the majority of cases. Its highly heterogeneous genetic landscape and limited treatment options pose significant challenges for clinicians. Conventional chemotherapy often fails due to drug resistance and harmful systemic side effects, underscoring the urgent need for innovative therapeutic strategies. Recent groundbreaking research conducted by a distinguished group of scientists affiliated with prestigious institutions such as Peking University and the Affiliated Suzhou Hospital of Nanjing Medical University has revealed a novel approach targeting Aurora kinases to combat liver cancer, showing promise not only in halting tumor growth but also in reprogramming cancer cells toward a more differentiated, less aggressive state.
Aurora kinases, consisting mainly of Aurora A and Aurora B isoforms, are critical serine/threonine kinases that play indispensable roles in mitosis and chromosome stability. Abnormal activation or overexpression of these kinases has been linked to tumorigenesis in various cancer types, positioning them as attractive candidates for targeted inhibition. While their involvement in cell cycle regulation is well documented, their impact on cellular differentiation, particularly in liver cancer, has remained inadequately explored. The latest study pioneers this avenue by evaluating whether inhibition of Aurora kinases could not only suppress uncontrolled cell proliferation but also restore hepatic differentiation in malignant liver cells.
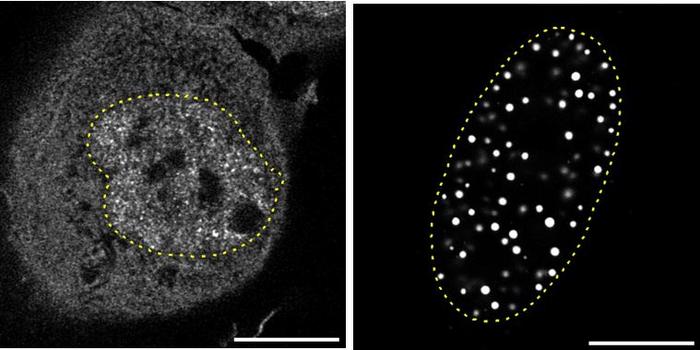
The researchers employed potent Aurora kinase inhibitors, chiefly Alisertib and ENMD-2076, to assess their therapeutic potential in HCC models. Employing a multifaceted experimental design, the team conducted cellular proliferation assays alongside comprehensive transcriptomic profiling of gene expression patterns in treated liver cancer cells. Their findings demonstrated that these inhibitors effectively arrested cell division, confirming the expected anti-mitotic effect. More importantly, the treatment induced a substantial upregulation of genes related to hepatic differentiation, indicating a shift in cancer cells toward a more mature and functionally competent phenotype typical of normal hepatocytes.
This phenotypic reversion was further validated by the simultaneous downregulation of malignancy-associated markers. The dual effect — suppression of proliferative capacity coupled with promotion of differentiation — is particularly noteworthy because it suggests a durable therapeutic benefit beyond mere cytotoxicity. The investigators observed that these differentiation-associated traits persisted for several days after cessation of drug exposure, implying a long-lasting reprogramming of cancer cell identity. This durability is critical in circumventing common issues such as tumor relapse and resistance development, which often undermine conventional therapies.
Mechanistic insights provided by transcriptomic analyses revealed that Aurora kinase inhibitors orchestrate their effects through a bifurcated mechanism. First, they directly interfere with mitotic progression, thereby curtailing unchecked tumor cell division. Second, and perhaps more intriguingly, they activate a network of hepatic transcription factors and metabolic genes that together foster the maturation and functional restoration of malignant cells. This dual action disrupts the malignant phenotype while reinstating physiological properties lost during carcinogenesis, offering a novel therapeutic paradigm that harmonizes tumor suppression with tissue homeostasis.
The implications of these findings extend far beyond liver cancer alone. Aurora kinases are ubiquitously expressed and implicated in the progression of multiple cancer types, suggesting that the concept of differentiation-based targeted therapy could revolutionize cancer treatment more broadly. Transitioning from strategies focused solely on cytotoxic eradication to those emphasizing induction of cellular differentiation offers potential advantages including reduced systemic toxicity and diminished likelihood of resistance. Such an approach aligns therapeutics more closely with normal biological processes, fostering a more sustainable and less deleterious intervention strategy.
Alisertib and ENMD-2076, the Aurora kinase inhibitors featured in the study, have already undergone prior clinical evaluations demonstrating favorable pharmacokinetic and safety profiles in various cancers, which accelerates their potential clinical translation for HCC treatment. Their established tolerability could facilitate swift progression into trials assessing their efficacy as differentiation-inducing agents in liver cancer patients. Additionally, these inhibitors could be integrated into combination regimens alongside existing cytotoxic drugs or emerging immunotherapies to exploit potential synergistic effects, thereby broadening the scope and effectiveness of liver cancer management.
Despite the promising preclinical results, the authors rightly emphasize the necessity for rigorous clinical investigations. Determining the optimal dosing regimens to maximize therapeutic benefits while minimizing adverse effects is paramount. Moreover, identifying biomarkers predictive of patient responsiveness to Aurora kinase inhibitors will be crucial for personalizing treatment and ensuring maximal efficacy. Such stratification could spare non-responders from unnecessary toxicity and tailor interventions to those most likely to achieve durable clinical benefit from differentiation-based therapies.
The study also enriches the understanding of the intricate molecular crosstalk between cell cycle regulation and cellular differentiation. By elucidating how cell division controllers like Aurora kinases interface with differentiation pathways, researchers gain valuable insight into cancer biology that transcends therapeutic applications. This knowledge not only informs drug development but also enhances the conceptual framework underlying tumor development and progression, potentially influencing diagnostic and prognostic strategies in liver cancer and beyond.
Importantly, the durability of differentiation induction by Aurora kinase inhibitors distinguishes this therapeutic strategy from transient cytostatic effects commonly seen with traditional agents. The sustained phenotypic shift observed suggests that cancer cells could be effectively ‘re-educated’ to regain functional characteristics, thereby reducing their malignancy and propensity for metastasis. This landmark feature highlights not just suppression but a partial reversal of oncogenic transformation, opening possibilities for long-term disease control or remission.
While many targeted therapies focus on blocking specific oncogenic signals, this study proposes a more nuanced approach centered on restoring lost cellular identity. By promoting maturation rather than mere destruction, differentiation-based therapies may mitigate systemic toxicity and improve quality of life in liver cancer patients. Furthermore, by addressing the underlying cell fate alterations inherent in oncogenesis, these interventions may offer a solution to the pervasive challenge of therapeutic resistance that plagues current treatment paradigms.
The translation of these findings into clinical practice holds great promise for improving the prognosis of patients diagnosed with HCC, a cancer with notoriously poor survival rates. With Aurora kinase inhibitors poised as viable candidates for differentiation therapy, future clinical trials are anticipated to establish their role within the therapeutic armamentarium. Should these studies validate the preclinical evidence, a new class of differentiation-based treatments may emerge, reshaping liver cancer therapy and offering renewed hope to patients worldwide.
In conclusion, this pioneering research convincingly demonstrates that Aurora kinase inhibition in liver cancer encompasses a dual mechanism: decisive arrest of tumor proliferation coupled with induction of a beneficial differentiation program. This strategy transcends traditional cytotoxic approaches, potentially revolutionizing HCC treatment by converting malignant cells back toward a more normal hepatic lineage. As the field moves forward, these insights offer a beacon of optimism that effective, durable, and safer liver cancer therapies are within reach.
—
Araştırma Konusu:
Aurora kinase inhibitörleri aracılığıyla karaciğer kanseri tedavisinde hücresel farklılaşmanın indüksiyonu ve tümör büyümesinin baskılanması.
Doi Referans:
10.1007/s11427-023-2795-2
Web References:
http://dx.doi.org/10.1007/s11427-023-2795-2
Anahtar Kelimeler:
Aurora kinazları, karaciğer kanseri, hepatoselüler karsinom, hücresel farklılaşma, Alisertib, ENMD-2076, hedefe yönelik tedavi, kanser biyolojisi, tümör proliferasyonu, karaciğer gen ekspresyonu