Now Reading: FLT3LG, Akciğer Adenokarsinomunda İmmün Yanıtı Güçlendiriyor
-
01
FLT3LG, Akciğer Adenokarsinomunda İmmün Yanıtı Güçlendiriyor
FLT3LG, Akciğer Adenokarsinomunda İmmün Yanıtı Güçlendiriyor
Immunotherapy has revolutionized the treatment paradigm for non-small cell lung cancer (NSCLC), particularly lung adenocarcinoma (LUAD), yet challenges remain in overcoming resistance and identifying patients most likely to benefit. A recent comprehensive study unravels the pivotal role of FLT3 ligand (FLT3LG) in shaping the tumor immune microenvironment and modulating the response to anti-PD-1 therapy, potentially charting new territory in precision oncology and immunotherapeutic efficacy.
Lung adenocarcinoma continues to be a leading cause of cancer-related mortality worldwide despite advances in targeted therapy and immunotherapy. The advent of immune checkpoint inhibitors targeting PD-1/PD-L1 pathways has extended survival and improved outcomes for subsets of patients. However, therapeutic resistance, both primary and acquired, limits the overall benefit of anti-PD-1 agents. This predicament underscores the urgency to discover reliable biomarkers that predict response and novel modulators that enhance therapeutic effectiveness.
In this context, FLT3LG emerges as a promising candidate with dual diagnostic and prognostic potential. FLT3LG, a growth factor known to regulate the development and function of dendritic cells, has been implicated in multiple cancer types, but its explicit role in LUAD remained obscure until now. Leveraging high-throughput bioinformatics analyses, the research delineates how FLT3LG expression correlates strongly with immune activity within the tumor milieu.
The study comprehensively analyzed clinical datasets revealing that elevated FLT3LG expression in LUAD patients associates with significantly improved prognosis. Molecular profiling emphasized that genes tightly co-expressed with FLT3LG cluster predominantly in immune-related signaling pathways, suggesting that FLT3LG is integrally linked to orchestrating antitumor immunity. These gene networks predominantly involve T cell activation, antigen presentation, and natural killer (NK) cell-mediated cytotoxicity.
An intriguing aspect uncovered by the research is the substantial positive correlation between FLT3LG levels and the infiltration of key immune effector cells—including CD4+ helper T cells, CD8+ cytotoxic T lymphocytes, and NK cells—in lung adenocarcinoma tissues. Increased immune infiltration is a hallmark of “hot” tumors, which typically respond better to immunotherapy. Thus, FLT3LG may serve as a marker of tumor immunogenicity.
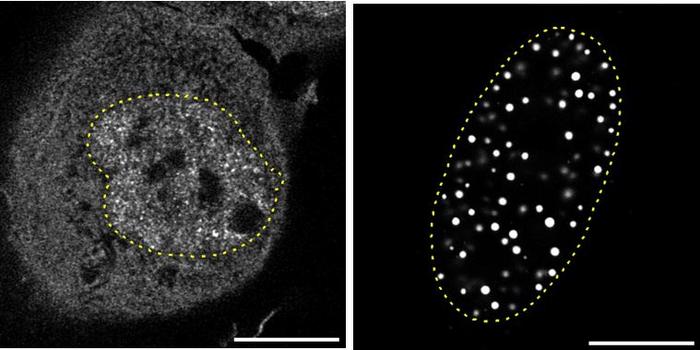
To validate these bioinformatics findings, the investigators employed murine models with subcutaneous lung adenocarcinoma grafts. Through FLT3LG overexpression, they demonstrated amplified immune cell accumulation within the tumor microenvironment under anti-PD-1 antibody treatment. This enhanced immune presence translated into a reinforced antitumor response and improved therapeutic efficacy compared to controls. These preclinical results provide compelling evidence that FLT3LG is more than a passive biomarker—it actively facilitates anti-PD-1 therapy effectiveness.
The experimental approach included sophisticated flow cytometry and immunohistochemistry technologies to quantitatively analyze immune cell populations and tumor tissue protein expression. These methodologies confirmed the increased presence of T cell markers such as CD4 and CD8a concomitant with FLT3LG elevation, underscoring its immunomodulatory role. Furthermore, serum assays in LUAD patients detected FLT3LG levels, indicating the feasibility of minimally invasive biomarker monitoring in clinical settings.
The therapeutic implications of these findings are profound. Beyond its diagnostic and prognostic utility, FLT3LG could be harnessed therapeutically to sensitize tumors to PD-1 blockade, overcoming immune evasion mechanisms characteristic of resistant lung adenocarcinomas. This novel strategy aligns with precision medicine’s goal of tailoring interventions based on molecular and immune profiling.
Moreover, the study enriches the growing understanding of the tumor microenvironment’s complexity. It highlights how modulating dendritic cell growth factors like FLT3LG may pivot the balance from immunosuppression to immune activation, thereby reinstating effective antitumor surveillance. Such insights pave the way for combinatory treatment protocols merging growth factor modulation with immune checkpoint inhibition.
FLT3LG’s role extends to acting as a sentinel of immune infiltration status, providing clinicians a biomarker to stratify patients. Those exhibiting high FLT3LG might represent an immune “hot” phenotype, predicting favorable responses to immunotherapy, whereas low expression could denote immune desertification and poor outcomes. Consequently, implementing FLT3LG screening in clinical workflows could optimize patient selection.
Critically, this research opens avenues for exploring FLT3LG-targeted agents or recombinant proteins as adjuncts to existing immunotherapies. Enhancing FLT3LG activity within tumors might recruit and activate antigen-presenting cells and effector lymphocytes, mitigating resistance and amplifying immune checkpoints’ blockade effects.
Future studies will no doubt delve into the mechanistic underpinnings of FLT3LG’s interaction with immune subsets, the kinetics of its expression during treatment, and its predictive power across diverse patient cohorts. Additionally, clinical trials assessing FLT3LG modulation may redefine lung adenocarcinoma management by bridging biomarker discovery and therapeutic innovation.
In conclusion, the nuanced investigation into FLT3LG in lung adenocarcinoma elucidates a critical axis influencing immune cell infiltration and anti-PD-1 therapy success. These findings herald a new frontier where molecular immunology informs both prognosis and personalized therapeutic approaches, promising improved patient outcomes in a notoriously challenging malignancy.
This landmark study reshapes our conceptual framework of tumor-immune dynamics and underscores the extraordinary potential latent within immune growth factors. As immunotherapy continues to evolve, integrating FLT3LG insights could transform lung cancer care, offering renewed hope for patients worldwide battling this formidable disease.
**Subject of Research:** FLT3LG’s role in immune cell infiltration and anti-PD-1 therapy response in lung adenocarcinoma
**Article Title:** FLT3LG modulates the infiltration of immune cells and enhances the efficacy of anti-PD-1 therapy in lung adenocarcinoma
**Article References:** Zhao, F., Bai, H., Liu, Y. et al. FLT3LG modulates the infiltration of immune cells and enhances the efficacy of anti-PD-1 therapy in lung adenocarcinoma. BMC Cancer 25, 831 (2025). https://doi.org/10.1186/s12885-025-14220-x
**Image Credits:** Scienmag.com
**DOI:** https://doi.org/10.1186/s12885-025-14220-x
**Keywords:** anti-PD-1 therapy, lung adenocarcinoma, bioinformatics, cancer immunology, immunotherapy resistance, FLT3LG, immune cell infiltration, tumor microenvironment, non-small cell lung cancer, prognostic biomarker, therapeutic response