Now Reading: Çocukluk Çağı Beyin Tümörleri Uzmanlaşmış Sinir Hücrelerinde Erken Gelişiyor
-
01
Çocukluk Çağı Beyin Tümörleri Uzmanlaşmış Sinir Hücrelerinde Erken Gelişiyor
Çocukluk Çağı Beyin Tümörleri Uzmanlaşmış Sinir Hücrelerinde Erken Gelişiyor
Medulloblastoma stands as one of the most formidable and common malignant brain tumors afflicting children and adolescents worldwide, originating predominantly in the cerebellum, the brain region coordinating movement and balance. Despite advances in pediatric oncology, the aggressive nature of medulloblastomas, especially subgroups three and four, complicates treatment and often leads to a poor prognosis. These tumors exhibit rapid growth, invasion into surrounding tissues, and a troubling propensity to metastasize, underscoring the urgent need for deeper biological understanding and innovative diagnostic strategies.
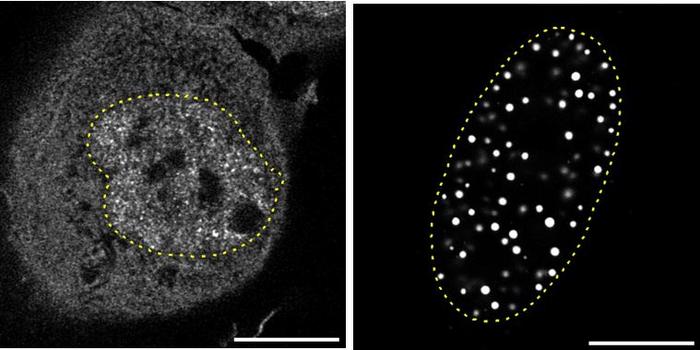
A pioneering study recently conducted by an interdisciplinary team from the Hopp Children’s Cancer Center Heidelberg (KiTZ), the German Cancer Research Center (DKFZ), and Heidelberg University Hospital (UKHD) has shed new light on the developmental timeline and cellular origins of the most aggressive medulloblastoma subtypes. Utilizing cutting-edge single-cell genetic analyses, the researchers meticulously profiled thousands of individual tumor cells from pediatric patients. This approach transcends traditional bulk tumor analysis, offering unprecedented resolution into the genetic heterogeneity and evolutionary history embedded within the tumor mass.
By leveraging single-cell sequencing technology, the scientists reconstructed the chronological sequence of genetic alterations within the tumor cells. This reconstruction revealed a critical bifurcation between “early” and “late” mutations, implying distinct phases in tumorigenesis. Early chromosomal abnormalities, characterized mainly by gains or losses of whole chromosomes or large chromosomal arms, appear to initiate tumor formation. These early alterations uniquely affect precursor cells known as unipolar brush cells (UBCs), specialized neurons in the cerebellum. Intriguingly, these UBC precursor cells are present and developing from the first trimester of pregnancy through the infant’s first year of life, indicating a prenatal window of tumor initiation.
Further analysis suggested that these large-scale chromosomal rearrangements occur largely by chance as early stochastic events within the unipolar brush cell lineage. These aberrations precede the clinical manifestation of the tumor by many years, highlighting a protracted latency period between tumor initiation and detection. This finding challenges existing paradigms which largely attribute tumorigenesis to oncogene activation alone, repositioning chromosomal instability as the foundational trigger.
Subsequent tumor progression appears to hinge on genetic changes later in tumor development—specifically, amplifications or mutations in cancer-driving genes such as MYC, MYCN, and PRDM6. These gene alterations emerge only in later cancer cell populations and correlate strongly with the tumor’s aggressive behavior, advancing rapid growth, metastatic potential, and resistance to therapy. The study thus decouples oncogene aberrations from tumor initiation, positioning them as critical for progression rather than the genesis of the tumor.
Lead researcher Lena Kutscher emphasized the clinical potential of these findings, noting that if early chromosomal changes can be detected through minimally invasive methods—such as circulating tumor DNA fragments in blood—early diagnosis in newborns or infants might become a possibility. Early detection could revolutionize treatment approaches, enabling interventions before tumor progression and metastasis, thereby improving survival and quality of life.
These insights derive from a collaboration involving multiple cutting-edge institutions: KiTZ, a specialized pediatric oncology research center combining clinical care and experimental science; DKFZ, Germany’s largest biomedical cancer research institute renowned for translational research; and UKHD, one of Europe’s leading academic medical centers committed to integrating research and patient care. Together, they embody a comprehensive “bench to bedside” approach, rapidly transforming laboratory discoveries into clinical innovations.
Medulloblastoma subgroups three and four have long posed diagnostic and therapeutic challenges due to their genetic complexity and aggressive clinical course. This study’s single-cell analysis provides a refined genetic map of these tumors, underscoring the importance of unipolar brush cells as the cell of origin. Understanding the timing and nature of chromosomal changes opens new avenues for research, including the development of biomarkers for early screening and potential preventive strategies.
Furthermore, the study accentuates the dynamic and evolving nature of cancer genomes within individual tumors. Tumor heterogeneity—previously a vexing obstacle in oncology—can now be parsed at single-cell resolution, revealing the subclonal architecture and temporal order of mutational events guiding both tumor initiation and progression. Such granularity is indispensable for designing therapies that target not only the dominant tumor clones but also the emergent resistant subpopulations.
This research highlights a crucial distinction in cancer biology: tumor initiation is often driven by chromosomal instability and large-scale genomic rearrangements, while oncogenes propel proliferation and metastatic competence. This dual-phase model calls for a shift in therapeutic targeting paradigms, suggesting that early intervention might require countering global genomic instability, whereas established tumors might respond better to targeted oncogene inhibition.
The implication that medulloblastomas initiate as early as the first trimester of pregnancy to infancy carries profound scientific and ethical considerations. It implicates prenatal and perinatal developmental windows as critical periods for tumorigenic risk and may influence future screening protocols and counseling in high-risk families.
In conclusion, this landmark study not only advances our understanding of medulloblastoma biology but also ignites optimism for earlier diagnosis and more tailored treatments. The ability to detect early chromosomal changes could spark a paradigm shift in pediatric oncology, aiming to intercept tumors at their nascent stages before malignant transformation consolidates. As research continues, integrating single-cell genomics into clinical practice promises to unravel the complexities of childhood brain tumors and pave the way for improved outcomes and survival rates.
**Subject of Research:** Human tissue samples
**Article Title:** Oncogene aberrations drive medulloblastoma progression, not initiation
**News Publication Date:** 7-May-2025
**Doi Referans:** 10.1038/s41586-025-08973-5
**Anahtar Kelimeler:** aggressive cancer subgroups, central nervous system tumors, cerebellum tumor growth, childhood brain tumors, genetic analysis of tumors, genetic characterization of tumors, Hopp Children’s Cancer Center research, medulloblastoma research, pediatric cancer treatment challenges, pediatric oncology advancements, single-cell analysis in oncology, tumor development history