Now Reading: Önceden Tedavi Görmüş Akciğer Kanserlerinde HER2 Hedefli Tedavi Ümit Vaat Ediyor
-
01
Önceden Tedavi Görmüş Akciğer Kanserlerinde HER2 Hedefli Tedavi Ümit Vaat Ediyor
Önceden Tedavi Görmüş Akciğer Kanserlerinde HER2 Hedefli Tedavi Ümit Vaat Ediyor
A novel therapeutic breakthrough has emerged in the treatment landscape of HER2-mutant non-small cell lung cancer (NSCLC), a historically difficult-to-treat subtype characterized by resistance to standard therapies and poor clinical outcomes. Researchers at The University of Texas MD Anderson Cancer Center have reported promising clinical trial results of zongertinib, an oral HER2-selective tyrosine kinase inhibitor developed by Boehringer Ingelheim. These findings denote a significant advance in targeted lung cancer treatment, particularly for those patients with specific HER2 mutations and previous treatment failures.
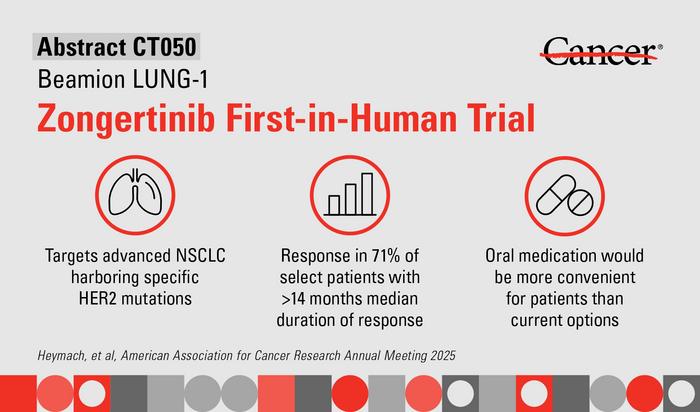
Zongertinib’s clinical efficacy was highlighted in updated data from the Phase Ia/Ib Beamion LUNG-1 trial, presented at the 2025 American Association for Cancer Research (AACR) Annual Meeting. This investigation focused on advanced and metastatic HER2-mutant NSCLC patients who had undergone prior therapy. Impressively, the therapy achieved a remarkable objective response rate (ORR) of 71% in patients with HER2 tyrosine kinase domain (TKD) mutations, signaling a profound tumor shrinkage effect far surpassing existing therapeutic responses in this patient cohort.
Beyond initial tumor response, the durability of zongertinib’s benefits demonstrated clinical relevance. The median duration of response (DOR) reached 14.1 months, while progression-free survival (PFS) extended to 12.4 months. These metrics underscore not only the drug’s ability to induce remission but also its potential to sustain tumor control over a meaningful timespan. Such sustained disease control marks a pivotal development given the aggressive nature and rapid progression commonly observed in HER2-mutant lung cancers.
A defining feature of zongertinib lies in its mechanism of action as a highly selective inhibitor targeting the HER2 receptor, while sparing the closely related epidermal growth factor receptor (EGFR). This selectivity reduces off-target toxicities associated with broader tyrosine kinase inhibitors, an advantage clearly reflected in the safety profile observed during the Beamion LUNG-1 trial. Severe adverse events (grade 3 or higher) were reported in only 17% of patients, predominantly consisting of manageable gastrointestinal and dermatologic side effects such as diarrhea and rash.
This improved tolerability contrasts with the only FDA-approved treatment currently available for this indication, trastuzumab deruxtecan (T-DXd), an antibody-drug conjugate administered intravenously. While effective, T-DXd carries a risk of severe, potentially fatal interstitial lung disease, an issue not observed with zongertinib during the trial. The oral delivery of zongertinib further enhances patient convenience, offering a once-daily pill regimen that may improve adherence and quality of life compared to intravenous therapies requiring clinical visits.
The Beamion LUNG-1 study additionally explored efficacy across different mutation subtypes and patient histories. Cohort 3 included 20 patients harboring non-TKD HER2 mutations, yielding an ORR of 30%, although duration and progression data for this group remain immature. Cohort 5, comprised of 31 patients previously treated with HER2-targeted antibody-drug conjugates, demonstrated an ORR of 48%, with a median DOR and PFS of 5.3 and 6.8 months, respectively. These findings suggest zongertinib retains activity even after resistance to prior HER2-targeted therapies, offering a vital treatment option for heavily pretreated populations where cross-resistance has limited options.
Such data hint at a unique capacity of zongertinib to overcome resistance mechanisms inherent in prior treatments, expanding its therapeutic scope. The ability to induce responses in both treatment-naïve and refractory patients emphasizes the drug’s versatility and addresses an unmet clinical need in HER2-mutant NSCLC, a diverse and biologically complex subtype often excluded from broader lung cancer therapeutic advances.
Zongertinib’s breakthrough status was recognized with a Food and Drug Administration (FDA) breakthrough therapy designation and priority review earlier in 2025, accelerating the pathway toward potential regulatory approval. These regulatory incentives were granted based on interim trial data that established zongertinib as a promising candidate surpassing existing therapeutic benchmarks for efficacy and safety in this difficult-to-treat patient population.
Anticipating these regulatory milestones, a Phase II randomized clinical trial is now underway to compare zongertinib head-to-head against the current standard of care, aiming to validate its superiority and further solidify its clinical role. Concurrently, a first-line treatment trial (Beamion LUNG-2) is recruiting patients to explore its use in earlier disease stages, potentially expanding the drug’s impact beyond previously treated, advanced cases.
Furthermore, researchers are investigating combination strategies pairing zongertinib with other targeted agents or immunotherapies, leveraging its specificity to enhance synergistic antitumor effects. Exploration of zongertinib across various tumor types harboring HER2 alterations—beyond NSCLC—also underscores a burgeoning research front in precision oncology, where mutation-driven therapies tailor individualized treatment plans.
This pivotal research was supported financially by Boehringer Ingelheim with substantial academic oversight from MD Anderson’s thoracic oncology team, notably led by Dr. John Heymach, M.D., Ph.D. Dr. Heymach characterized the therapeutic results as “unprecedented” and emphasized the novelty of offering patients an effective, safe, and convenient oral option in a domain previously devoid of such advancements.
Ultimately, zongertinib represents a new chapter in precision medicine for lung cancer, where the convergence of molecular understanding and innovative drug design yields meaningful clinical progress. For patients battling HER2-driven NSCLC, this oral therapy brings renewed hope and aligns with a growing movement toward less invasive, more patient-centric cancer treatments. The anticipation surrounding its continued clinical evaluation and potential approval reflects a broader paradigm shift in oncology toward highly selective, mutation-specific interventions with improved safety, efficacy, and quality of life.
—
**Subject of Research**: HER2-targeted therapy for advanced HER2-mutant non-small cell lung cancer (NSCLC)
**Article Title**: Zongertinib Demonstrates Promising Efficacy and Safety in HER2-Mutant Lung Cancer: Results from the Beamion LUNG-1 Trial
**Haberin Yayın Tarihi**: April 28, 2025
**Resim Credits**: The University of Texas MD Anderson Cancer Center
**Anahtar Kelimeler**: Boehringer Ingelheim, oncology research, convenience in cancer therapy, emerging cancer treatment options, FDA priority review, cancer drugs, HER2-targeted therapy, innovative lung cancer therapies, oral pill cancer treatment, Phase II clinical trials, poor prognosis HER2-mutant cancers, previously treated lung cancers, resistance to standard therapies, zongertinib lung cancer treatment